Early Detection Initiative

Our Early Detection Initiative is undertaken in collaboration with the research community to improve early diagnosis of pancreatic cancer. Our Initiative provides increased focus and funding for projects that will allow the earlier detection of pancreatic cancer.
“Currently, there is no established way to test or find pancreatic cancer early. Only around 10% of pancreatic cancers are diagnosed before they spread. However, if detected early, long-term (5 years +) survival rates are 10 times higher. We believe that early diagnosis offers the greatest hope to triple survival rates by 2030,”
~ Michelle Stewart, CEO PanKind
Early Detection Research
Since our inception, PanKind have been committed to funding impactful research that furthers the understanding of pancreatic cancer, including projects to detect the cancer earlier and provide better treatment options to improve survival outcomes.
Our Early Detection Initiative demonstrates our increased focus and commitment to driving research that will quickly progress early detection for pancreatic cancer.
Pancreatic Cancer Early Diagnosis Group
Collaborative led by Prof Rachel Neale, QIMR-Berghofer Medical Research Institute
This project focuses on detecting pancreatic cancer earlier before symptoms develop so they have a greater chance of survival. General population screening is not feasible, partly due to the low incidence of pancreatic cancer in the general population, so we need to focus on people that are at a markedly higher risk of developing pancreatic cancer and create a surveillance program. The team is doing this by harnessing the power of national large-scale data linkage and modern machine learning methods to identify a cohort of patients who would benefit from surveillance. The second phase of this project will focus on reducing delays in diagnosis.
Jreissati Family Pancreatic Cancer Centre in Collaboration with Royal Melbourne
Collaborative led by A/Prof Andrew Metz, Epworth HealthCare, Royal Melbourne Hospital
This project centres on the development of a novel clinical screening tool in people with diabetes and chronic pancreatitis for the early detection of pancreatic cancer.
This project aims to find a correlation between the diagnosis of type 3c diabetes and the onset of pancreatic cancer. Both principal investigators are long term colleagues and have together created an extensive database over 20 years comprising >10,000 patients with diabetes and/or chronic pancreatitis treated at the Royal Melbourne and Epworth Hospitals.
Given over 96,000 people are diagnosed with diabetes annually in Australia it would be cost prohibitive to screen all people for pancreatic cancer, however determining diabetes disease subtypes such as classifying with features of type 3c diabetes along with weight categories, age, family history of neoplasia and blood biomarkers, may provide us with a novel and cost effective screening application.
This research is novel and aims to challenge the status quo by altering diagnostic paradigms to improve outcomes for people with pancreatic cancer.
Australian Pancreatic Cancer Genome Initiative
Garvan Institute of Medical Research
The APGI's ongoing mission is to further develop the existing world-class resource comprising biological samples and comprehensive information on pancreatic cancer patients from diagnosis onwards, including clinical, genomic and outcome data and to use this resource to improve pancreatic cancer care in the era of personalised medicine. Biospecimens are materials taken from the human body, such as tissue or blood, that can be used for cancer diagnosis and analysis. When patients have a biopsy or surgery often a small amount of the specimen removed can be stored and used for research. Biospecimens contain an extraordinary amount of biological information, written in the language of cells, genes and proteins. Researchers can then frame questions that will be answered by looking at these biospecimens, for example, they often use the biospecimen to identify the biological characteristics of cancer cells over time, and then correlate those patterns with the clinical picture - and investigate how different patients experience progression of the disease.
Development of a non-invasive method of early detection for pancreatic cancer patients using nanotechnology
Dr Ying Zhu, The University of New South Wales, 2019 Innovation grant
Dr Zhu and her team will use their grant to develop an integrated and small device based on nanotechnology for rapid and sensitive exosome analysis.
The team will define a set of biomarkers that can differentiate between cancer and non-cancer subjects from cells and plasma carrying early signs of human pancreatic cancer. This novel technology will also be applicable for doctors monitoring the development and customising the treatment of a patient’s tumour.
The team will aim to develop a blood test to detect pancreatic cancer in the early stages. The blood test will detect exosomes, which are nanosized fragments released by cancer cells. Exosomes are important for communicating messages and transporting materials between cells. Exosomes have been identified as more accurate and promising biomarkers or biological clues for pancreatic cancer diagnosis.
A novel agent for imaging and treatment of pancreatic cancer
Prof John Hooper, The University of Queensland, 2019 Innovation Grant
Professor Hooper and his team will use this grant to develop new precision-medicine agents, called theranostics, for detection and treatment of the most common form of pancreatic cancer, pancreatic ductal adenocarcinoma (PDAC). These agents can accurately detect tumours using PET-CT imaging. They provide a powerful and selective way to deliver anti-cancer drugs to PDAC tumours, and would, therefore, improve the effectiveness of anti-cancer drugs whilst reducing their side-effects. Professor Hooper’s team has already successfully developed a lead theranostic agent that is very effective at detecting and treating pancreatic cancer in relevant pre-clinical models in the laboratory. The team will now bioengineer the lead theranostic so that it can be administered to patients for clinical testing, and then manufactured in quantities that are clinically useful and economically viable for production and marketing. Bioengineering will also allow the lead agent to be readily linked with a range of molecular imaging and anti-cancer drugs that are designed to best suit each PDAC patient.
A novel therapeutic target in pancreatic cancer: Implications for therapy and diagnosis
Prof Marco Falasca, Curtain University, 2016 Accelerator Grant
Project
The project team have discovered that a protein named GPR55 promotes pancreatic cancer growth. Their preliminary data demonstrates that a GPR55 inhibitor, in combination with a drug used in therapy, is able to increase survival in mice that develop pancreatic cancer. The project team’s plan is to identify more potent drug combinations to treat pancreatic cancer and also identify new marker that will enable to diagnose pancreatic cancer earlier.
Impact
In this research, the team identified a synergistic combination of gemcitabine and cannabidiol on pancreatic cancer models in vitro and in vivo. In addition, they have a stronger effect on tumorspheres compared to the corresponding parental tumour cells. The implications of these key discoveries are that a combination of cannabidiol and gemcitabine can be used in clinical trials and the major effect of extracts on pancreatic cancer tumorspheres suggests that they could be more efficacious in resistant tumours. The major objective of Professor Falasca’s work is to bring these discoveries to the clinical trial stages. The team is very close to reaching this outcome and are discussing with clinicians and a pharmaceutical company the details of the trials. In addition, this research stimulated many other research avenues such as the investigation of exosomes role in pancreatic cancer and the identification of novel targets such as ABCC3.
Circulating stromal cells (pancreatic stellate cells) and tumour cells in pancreatic cancer
Prof Minote Apte, The University of New South Wales, 2015 Innovation Grant
Project
The spread of cancer from the primary organ to distant sites involves the movement of clusters of cancer cells and stromal cells from the original tumour through the circulation. Prof Apte aims to characterise these cells so as to identify novel markers that would aid early diagnosis as well as facilitate assessment of treatment response and prognosis.
Impact
Prof Apte reported that the most important research finding produced by this Project funded by the PanKind thus far is that circulating pancreatic stellate cells can indeed be isolated in the blood of patients with pancreatic cancer. Furthermore, as these pancreatic stellate cells are likely important in the formation of metastases, Prof Apte’s findings open the door to a new avenue of research in the prevention of pancreatic cancer metastases. Prof Apte effectively isolated circulating tumour cells in the portal vein blood of pancreatic cancer carrying mice. She positively demonstrated that the number of circulating tumour cells in the portal vein is higher than that found in cardiac puncture samples and that the number of such cells in the portal vein correlates with the size of the tumour. Prof Apte has reported that this is also an important finding as it lends further strength to the concept of portal vein sampling in the clinical setting to maximise the probability of successfully obtaining circulating tumour cells for analysis.
“We thank the Foundation for supporting pancreatic cancer research. This Project has led to the first ever successful isolation of circulating pancreatic stellate cells in a pancreatic cancer patient as well as in a mouse model of pancreatic cancer. These findings open a new area of investigation for the prognostication and the treatment of pancreatic cancer.”
~ Prof Minote Apte
What is early detection?
“Early diagnosis of cancer focuses on detecting symptomatic patients as early as possible, so they have the best chance for successful treatment. When cancer care is delayed or inaccessible there is a lower chance of survival, greater problems associated with treatment and higher costs of care.”(1)
Why is early detection important?
People who have pancreatic cancer diagnosed in the early stages of disease have better outcomes. If pancreatic cancer is detected before it has grown too large or spread to other areas in the body, there are more treatment options available, including surgery.
Only around 10% of pancreatic cancers are diagnosed before they spread. However, if detected early, long-term (5 years +) survival rates are 10 times higher than if the cancer has already spread. It is important to remember that while survival statistics give an indication of prognosis on a population level scale, they cannot describe individual cases.(2,3)
Early detection also provides greater time and opportunity for patients to participate in research, accelerating discovery and treatment options. By detecting pancreatic cancer in its early stages, we can gain valuable insights that may pave the way for innovative treatments and prevention strategies.
Why is pancreatic cancer hard to diagnose?
- The symptoms of pancreatic cancer are not always obvious, and can change over time. It’s typical for symptoms only to appear once the cancer has spread or is large enough to affect nearby organs. Additionally, pancreatic cancer symptoms can manifest in diverse ways and may vary among patients.
- The pancreas is located deep within the body, so it can be hard to see or feel a tumour from a physical exam.
- Current diagnostic tests do not always detect small, pre-cancerous or early-stage tumours.
- While there are several diagnostic tests available, there is not yet a single standard test.
- There are no proven biological markers that can be used to determine if you have pancreatic cancer.
- As pancreatic cancer is a less common cancer, population-wide screening programs are not recommended, and researchers are yet to determine conclusively which are the best sections of the population to screen.
Providing renewed hope through early detection of pancreatic cancer
Ultimately, our Early Detection Initiative serves as a crucial step forward in actively combating pancreatic cancer. PanKind is committed to gathering researchers, healthcare professionals and the community to shape a future where early detection becomes the norm.
Early detection of pancreatic cancer presents immense benefits, from enabling timely intervention and achieving better outcomes, to advancing research efforts. With this initiative, we hope to increase survival rates significantly and offer renewed hope to patients and their families.
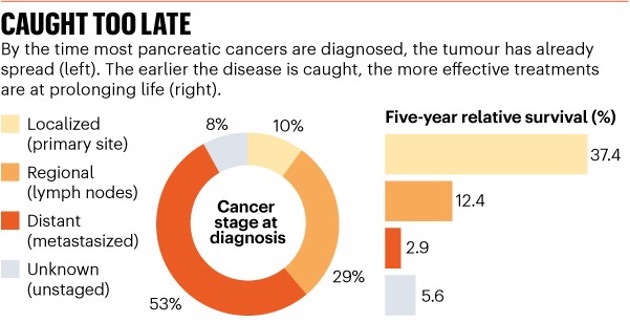
Source: US National Cancer Institute
Why is pancreatic cancer hard to diagnose?
- The symptoms of pancreatic cancer are not always obvious, come and go and can change over time.
- The pancreas is located deep within the body, so it can be hard to see or feel a tumour from a physical exam.
- Current diagnostic tests do not always detect small, pre-cancerous or early-stage tumours.
- While there are several diagnostic tests available, there is not yet a single standard test.
- There are no proven biological markers that can be used to determine if you have pancreatic cancer.
- As pancreatic cancer is a less common cancer, population-wide screening programs are not recommended, and researchers are yet to determine conclusively which are the best sections of the population to screen.
(1) World Health Organisation.
(2) American Cancer Society, Survival Rates for Pancreatic Cancer.
Always consult your doctor or health professional about any health-related matters. PanKind does not provide medical or personal advice and is intended for general informational purposes only. Read our full Terms of Use.
Thank you to the clinicians, researchers, patients, and carers who have helped us create and review our website information and support resources, we could not have done it without you.







